Our SCIP Compliance Solution
Companies placing an article containing an SVHC above 0.1% on the EU market are required to submit information to the SCIP database from January 5th, 2021 onwards. With the majority of complex products containing SVHCs above 0.1% w/w, this requirement will impact most importers to and manufacturers within the European Union.
iPoint is member of the ECHA IT User Group
As a official member of the IT User Group we provide input on the development of the system, share our ideas for improvement, analyse different scenarios and help to identify the areas where extra attention is needed in order to provide adequate support/guidance. Based on our extensive expert knowlegde we have developed a connector for the automated information exchange between ECHA’s SCIP database and iPoint Compliance.
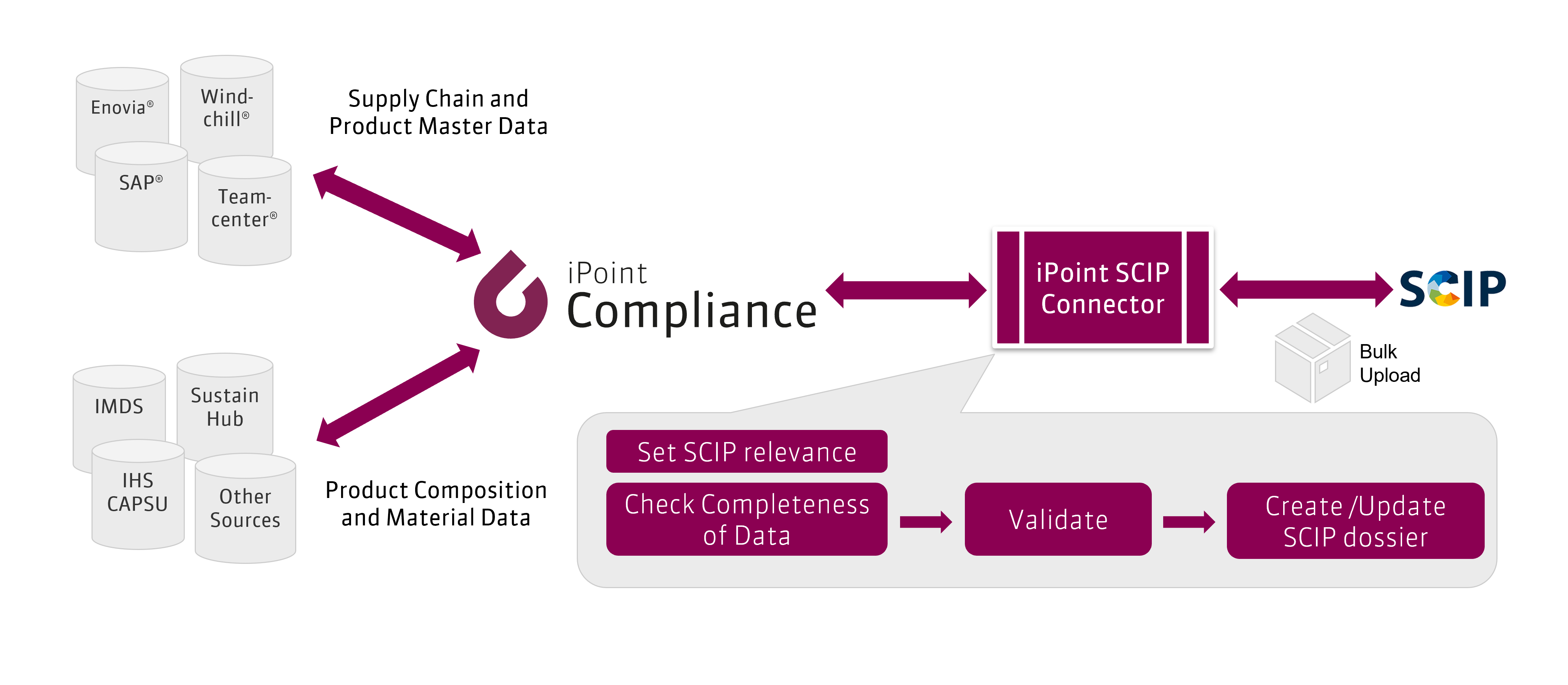
iPoint's SCIP Connector
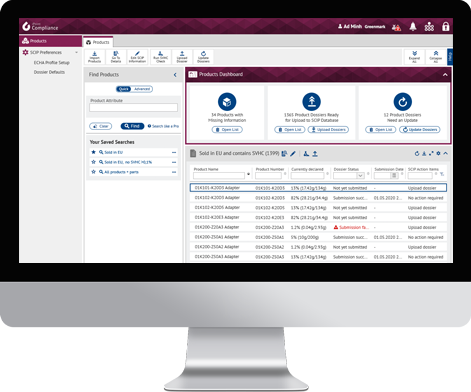
With the iPoint SCIP Connector we offer a system-to-system solution that allows a bulk upload of data to ECHA’s SCIP database.
- Management of your company’s one or more ECHA UUIDs required for SCIP submission
- Extensive options of default settings allowing easy initial SCIP reporting on the basis of existing data
- Management of product details and setting of SCIP relevance
- Dossier Creation, Validation and Upload

Collect
Analyze
Report
Discover the iPoint Suite

iPoint Compliance
Learn what works best for your company. In the video you will get the latest updates on the ECHA SCIP database and see a live demo of the officially released iPoint's SCIP Database Connector.

The Echa SCIP Database
FAQ
We have received a lot of questions on the SCIP Database. Here are the answers on the TOP 40 frequently asked questions.

SCIP Database Insights
White Paper
Are you ready for SCIP reporting? Be prepared for the challenges of the SCIP database
> Download the free white paper
What is the SCIP database?
With the revision of the Waste Framework Directive (EU) 2018/851, Article 9, the European Chemicals Agency ECHA was tasked with the implementation of a database collecting (and making available) information on articles containing Substances of Very High Concern (SVHC) above 0.1% by weight as specified by the Candidate list.
ECHA has published a document detailing the information requirements of the so-called SCIP database. Companies placing an article containing an SVHC above 0.1% on the EU market are required to submit information from January 5th, 2021 onwards.
SCIP stands for Substances of Concern In articles, as such or in complex objects (Products). Although the SCIP acronym encompasses “products”, ECHA explicitly avoids using the term in the description of its information requirements limiting it to “articles”. Mixtures and substances are not under consideration, information obligations exist for articles as such as well as articles in complex objects. (For a more detailed description see ECHA’s Guidance on requirements for substances in articles.)
Three key information constituents have to be provided
article identification
safe use information and
identification, location, and concentration of the Candidate list substance(s) contained
> For the latest updates on SCIP please visit our blog that we frequently update